Course Description
While this course is still ongoing, it serves as the capstone of the Thayer BE curriculum. The fall term mixes project planning and problem identification with a professionalism curriculum that ties together the technical engineering required to plan and execute a project with a liberal arts approach to understanding the ethics, inclusion, and impact that engineering necessitates.
Surgical Site Infection Pathway
UV Intraoperative Therapy Project Executive Summary
Problem Statement
Surgical Site Infections (SSIs) induce significant risk to surgical patient quality of life, financial wellbeing, and hospital length of stay. By determining the efficacy and safety of using 222 nm UV radiation to disinfect internal organs we strive to provide the necessary research for our sponsors, and others, to make operations safer and less expensive for the patient.
Innovation
The majority of scholarly attention has focused on utilizing 222 nm UV light for bacterial inactivation in food, water, or air. In such disinfection applications there is no need to optimize the energy administered by the UV source to kill the pathogens because so long as the pathogens die, the medium remains otherwise unimpacted. However, exposing subdermal flesh to UV light poses a risk of damaging patient cells and thus the dosage must be optimized such that it kills bacteria while leaving the patients and surgeons unharmed.
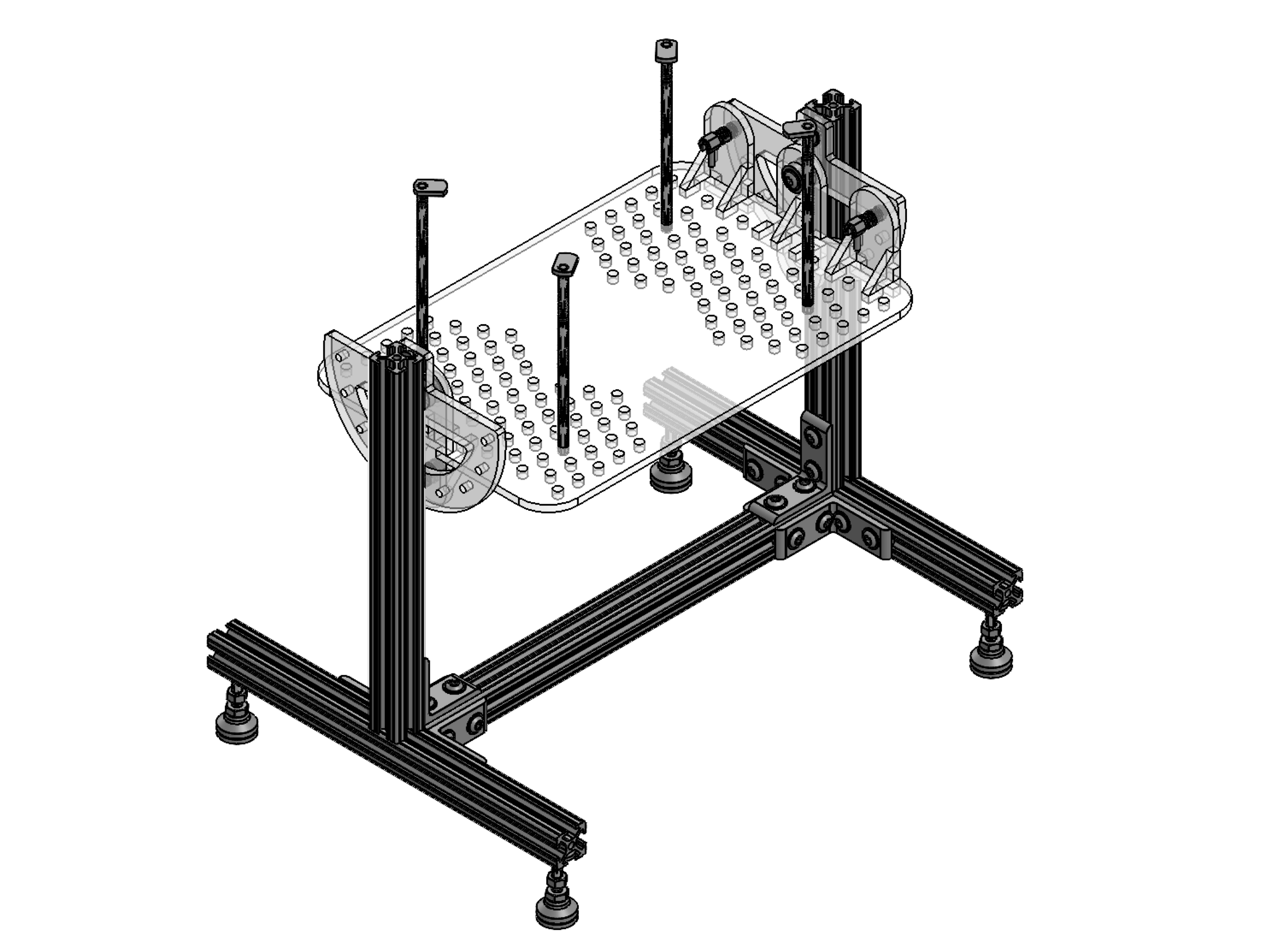
While research conducted on the safety of 222 nm UV light on mammalian skin has verified the technology’s safety for human eye and skin contact within moderate dosage levels, equivalent research does not exist for internal organs. Without this data, the viability of UV surgical site disinfection devices is unknown. Through our research we will characterize the sterilization efficacy of 222 nm light in a surgical context and investigate the safety limits of such radiation. Testing will be conducted on a selection of mouse organs which will be exposed to varied doses of UV light and analyzed for harmful DNA damage through an immunohistochemical assessment.
Value
Regardless of the findings of our testing, the research will prove useful to those developing UV clinical devices. If significant cellular damage is observed at low irradiances, the viability of UV disinfection devices will diminish, thereby saving the expenditure of valuable resources. However, should no cellular damage be observed at the high end of dosing, UV disinfection products would remain promising, and further investigations and development would be warranted.
Importantly, disinfection products that utilize 222 nm UV technology are effective against antibiotic-resistant pathogens, providing a major perk over antibiotics, and fighting an increasingly important issue in medicine. The successful implementation of this technology into a clinical device could prevent thousands of SSIs annually, saving many lives and reducing the monetary and physical resources expended by hospitals and patients.
Control Sample Imaging
Our 222 nm test exposure samples will be compared against known positive (254 nm wavelength, known to be carcinogenic) and negative (no UV exposure) control samples:
Negative Control Sample: No UV Exposure
Positive Control Sample: 254 nm Wavelength Exposure
Academic Deliverables
Project Proposal Presentation
This presentation was given by our team at the end of the first term to the review board which decides on the feasibility of a project. It gives a general outline of the problem which we are facing, the work we have done thus far, the context in which our product will be developed, and the plan we have for moving forward this winter.
Project Proposal Writeup
This writeup was given to the same review board as well as our sponsors and advisors and provides an in-depth technical report of the background research, metrics of success, technical plan, personal experience, and financial viability of our project.